Your Jaguar health fda approval images are ready. Jaguar health fda approval are a topic that is being searched for and liked by netizens today. You can Get the Jaguar health fda approval files here. Find and Download all royalty-free images.
If you’re searching for jaguar health fda approval pictures information linked to the jaguar health fda approval interest, you have pay a visit to the right site. Our site always provides you with hints for refferencing the highest quality video and image content, please kindly search and find more informative video articles and images that fit your interests.
Jaguar Health Fda Approval. FDA laboratory analysis confirmed that Jaguar Power contains sildenafil the active ingredient in the FDA-approved prescription drug Viagra used to treat erectile dysfunction. Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM. NASDAQJAGX today provided updates regarding ongoing investigator-initiated trials in non-HIV patient populations of crofelemer Mytesi the novel FDA-approved plant-based oral drug developed and marketed by Jaguars wholly-owned subsidiary Napo Pharmaceuticals Inc.
 Check It Out Http Recycle Tattoo Tattoos Small Tattoos Simple Tattoo Studio From pinterest.com
Check It Out Http Recycle Tattoo Tattoos Small Tattoos Simple Tattoo Studio From pinterest.com
NASDAQJAGX today provided updates regarding ongoing investigator-initiated trials in non-HIV patient populations of crofelemer Mytesi the novel FDA-approved plant-based oral drug developed and marketed by Jaguars wholly-owned subsidiary Napo Pharmaceuticals Inc. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM. Canalevia would be first and only FDA-approved plant-based medicine for working dogs that suffer from diarrhea. Jaguar Health in March applied for Emergency Use Authorization EUA from the US. Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi. FDAs approval of.
FDA Invites Jaguar Health to Expedite Submission of Regulatory Filing for Approval of Canalevia Crofelemer to Treat Chemotherapy-induced Diarrhea in Dogs Published Jul 20 2020 830AM EDT.
JAGX Stock Price News. Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi. NASDAQJAGX today announced that it has completed the filing of the New Animal Drug Application NADA to request the US. SAN FRANCISCO CA ACCESSWIRE November 30 2020 Jaguar Health Inc. Specifically it looks to develop plant-based prescription medicines. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM.

Marinol is an FDA-approved drug. Jaguar Health is also seeking FDA approval for Canalevia its canine-specific formulation of crofelemer for chemotherapy-induced diarrhea CID in dogs. JAGX will receive pre-clinical. Jaguar Health Reports Completed Filing Of New Animal Drug Application For Conditional Approval Of Canalevia-CA1 To Treat Chemo-Induced Diarrhea In Dogs. It is the only oral plant-based prescription medicine approved under FDA Botanical Guidance.
 Source: pinterest.com
Source: pinterest.com
Canalevia would be first and only FDA-approved plant-based medicine for working dogs that suffer from diarrhea. The company cites an addressable market of. Crofelemer is the active ingredient in Mytesi Jaguar Healths FDA-approved drug to treat noninfectious diarrhea in adult patients with HIVAIDS on antiretroviral therapy ART. Jaguar Health Inc. Jaguar Health Inc is up 1947 today after final submission of Canalevia filing for diarrhea in dogsThey await approval from the FDAs Center of Veterinary Medicine on their oral plant-based drug candidate.
 Source: jaguar.health
Source: jaguar.health
FDA Invites Jaguar Health to Expedite Submission of Regulatory Filing for Approval of Canalevia Crofelemer to Treat Chemotherapy-induced Diarrhea in Dogs Published Jul 20 2020 830AM EDT. Jaguar Health Completes Filing of New Animal Drug Application for Conditional Approval of Canalevia-CA1 Crofelemer to Treat Chemotherapy-Induced Diarrhea in Dogs Clearing. The company is also betting on Mytesi as a treatment for cancer therapy-induced diarrhea. SAN FRANCISCO CA ACCESSWIRE August 19 2020 Jaguar Health Inc. The company cites an addressable market of.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Napo EU SpA. Jaguars wholly owned subsidiary Napo Pharmaceuticals Inc currently markets a form of crofelemer Mytesi which is the only non-opioid oral plant-based medicine approved by the FDA for the. Crofelemer is the active ingredient in Mytesi Jaguar Healths FDA-approved drug to treat noninfectious diarrhea in adult patients with HIVAIDS on antiretroviral therapy ART. Jaguar also seeking approval to market Canalevia for exercise-induced diarrhea in dogs. Napo EU SpA.
 Source: pinterest.com
Source: pinterest.com
Jaguar Health Reports Completed Filing Of New Animal Drug Application For Conditional Approval Of Canalevia-CA1 To Treat Chemo-Induced Diarrhea In Dogs. Jaguar Health Receives Complete Letter from FDA for Last of Four Major Technical Sections for the Companys Application for Conditional Approval of Canalevia Crofelemer for Chemotherapy. Crofelemer is the active ingredient in Mytesi Jaguar Healths FDA-approved drug to treat noninfectious diarrhea in adult patients with HIVAIDS on antiretroviral therapy ART. Jaguar Health in March applied for Emergency Use Authorization EUA from the US. Jaguar Health Inc.
 Source: pinterest.com
Source: pinterest.com
It is the only oral plant-based prescription medicine approved under FDA Botanical Guidance. FDA Invites Jaguar Health to Expedite Submission of Regulatory Filing for Approval of Canalevia Crofelemer to Treat Chemotherapy-induced Diarrhea in Dogs Published Jul 20 2020 830AM EDT. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM. Food and Drug Administrations conditional approval to market Canalevia -CA1 crofelemer delayed-release tablets Jaguars oral plant-based. Jaguar Health Inc.
 Source: jaguarhealth.gcs-web.com
Source: jaguarhealth.gcs-web.com
Jaguar Health in March applied for Emergency Use Authorization EUA from the US. Jaguar Health in March applied for Emergency Use Authorization EUA from the US. Jaguar Health Reports Completed Filing Of New Animal Drug Application For Conditional Approval Of Canalevia-CA1 To Treat Chemo-Induced Diarrhea In Dogs. Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM.
 Source: investorsobserver.com
Source: investorsobserver.com
SAN FRANCISCO CA ACCESSWIRE November 2 2021 Jaguar Health Inc. The company cites an addressable market of. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM. About Jaguar Health Inc Napo Pharmaceuticals Inc. SAN FRANCISCO CA ACCESSWIRE July 2 2020 Jaguar Health Inc.
 Source: pinterest.com
Source: pinterest.com
Food and Drug Administration for Mytesi to treat similar symptoms in coronavirus patients who are also sometimes. Jaguar Health says it focuses on developing sustainably derived gastrointestinal products. Jaguar Health is also seeking FDA approval for Canalevia its canine-specific formulation of crofelemer for chemotherapy-induced diarrhea CID in dogs. Crofelemer is the active ingredient in Mytesi Jaguar Healths FDA-approved drug to treat noninfectious diarrhea in adult patients with HIVAIDS on antiretroviral therapy ART. Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi.
 Source: pinterest.com
Source: pinterest.com
Specifically it looks to develop plant-based prescription medicines. JAGX will receive pre-clinical. Specifically it looks to develop plant-based prescription medicines. NASDAQJAGX Jaguar or the Company and the Companys wholly-owned subsidiary Napo Pharmaceuticals Inc. FDA laboratory analysis confirmed that Jaguar Power contains sildenafil the active ingredient in the FDA-approved prescription drug Viagra used to treat erectile dysfunction.
 Source: pinterest.com
Source: pinterest.com
It is the only oral plant-based prescription medicine approved under FDA Botanical Guidance. SAN FRANCISCO CA ACCESSWIRE July 2 2020 Jaguar Health Inc. NASDAQJAGX Q3 2021 Earnings Conference Call November 15 2021 830 AM ET Company Participants Carol Lizak Chief Financial Officer Lisa Conte President and Chief. Jaguars wholly owned subsidiary Napo Pharmaceuticals Inc currently markets a form of crofelemer Mytesi which is the only non-opioid oral plant-based medicine approved by the FDA for the. Jaguar planning for launch of Canalevia for CID in dogs this DecemberCanalevia is the first and only oral plant-based prescription drug candidate for CID in dogsSAN FRANCISCO CA ACCESSWIRE September 23 2021 Jaguar Health Inc.
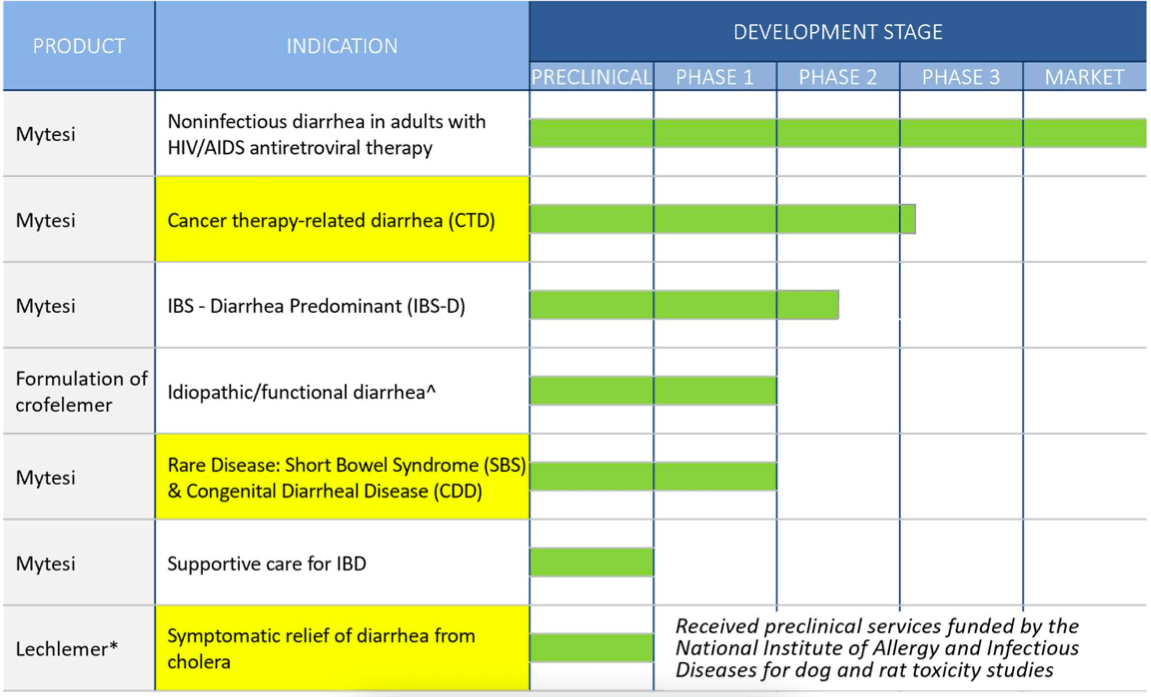 Source: seekingalpha.com
Source: seekingalpha.com
Napo announced today that Jaguar and Napo are planning to develop and commercialize crofelemer the Companys novel proprietary drug for an indication of. SAN FRANCISCO CA ACCESSWIRE July 2 2020 Jaguar Health Inc. Marinol is an FDA-approved drug. Napo EU SpA. SAN FRANCISCO CA ACCESSWIRE November 2 2021 Jaguar Health NASDAQJAGX today announced that it has completed the filing of the New Animal Drug Application NADA to request the US.
 Source: pinterest.com
Source: pinterest.com
JAGX Stock Price News. NASDAQJAGX surged by 4925 on Friday to close a tremendous first week of 2021. Jaguar Health Inc. Napo EU SpA. Jaguar Health Reports Completed Filing Of New Animal Drug Application For Conditional Approval Of Canalevia-CA1 To Treat Chemo-Induced Diarrhea In Dogs.
 Source: jaguarhealth.gcs-web.com
Source: jaguarhealth.gcs-web.com
NASDAQJAGX today provided updates regarding ongoing investigator-initiated trials in non-HIV patient populations of crofelemer Mytesi the novel FDA-approved plant-based oral drug developed and marketed by Jaguars wholly-owned subsidiary Napo Pharmaceuticals Inc. Is a commercial stage pharmaceuticals company focused on developing novel plant-based non-opioid and. Jaguar Health says it focuses on developing sustainably derived gastrointestinal products. NASDAQJAGX today announced that it was informed on September 21 2021 by the FDAs Center for Veterinary Medicine CVM. Food and Drug Administration for Mytesi to treat similar symptoms in coronavirus patients who are also sometimes.
 Source: pinterest.com
Source: pinterest.com
NASDAQJAGX today provided updates regarding ongoing investigator-initiated trials in non-HIV patient populations of crofelemer Mytesi the novel FDA-approved plant-based oral drug developed and marketed by Jaguars wholly-owned subsidiary Napo Pharmaceuticals Inc. Jaguar Health in March applied for Emergency Use Authorization EUA from the US. Specifically it looks to develop plant-based prescription medicines. SAN FRANCISCO CA ACCESSWIRE July 2 2020 Jaguar Health Inc. It is the only oral plant-based prescription medicine approved under FDA Botanical Guidance.
 Source: pinterest.com
Source: pinterest.com
Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi. Jaguar Health NASDAQJAGX gains FDA approval to market Canalevia crofelemer delayed-release tablets an oral plant-based prescription drug candidate for the treatment of exercise-induced. FDAs approval of. Jaguar Health says it focuses on developing sustainably derived gastrointestinal products. About Jaguar Health Inc Napo Pharmaceuticals Inc.
 Source: pinterest.com
Source: pinterest.com
Jaguar Health Reports Completed Filing Of New Animal Drug Application For Conditional Approval Of Canalevia-CA1 To Treat Chemo-Induced Diarrhea In Dogs. SAN FRANCISCO CA ACCESSWIRE November 2 2021 Jaguar Health NASDAQJAGX today announced that it has completed the filing of the New Animal Drug Application NADA to request the US. Canalevia would be first and only FDA-approved plant-based medicine for working dogs that suffer from diarrhea. About Jaguar Health Inc Napo Pharmaceuticals Inc. Napo EU SpA.
 Source: pinterest.com
Source: pinterest.com
Marinol is an FDA-approved drug. FDA laboratory analysis confirmed that Jaguar Power contains sildenafil the active ingredient in the FDA-approved prescription drug Viagra used to treat erectile dysfunction. Investors should note that Jaguar Health is currently hoping to receive further FDA approval for Mytesi. About Jaguar Health Inc Napo Pharmaceuticals Inc. Jaguar Health says it focuses on developing sustainably derived gastrointestinal products.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title jaguar health fda approval by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






